Leading Questions
Orgo questions? Forgetaboutit!
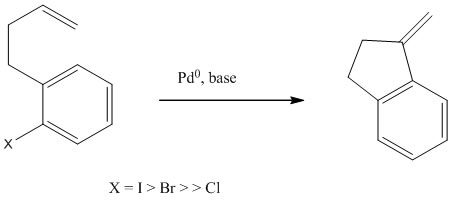
What is the Intramolecular Heck Reaction?
The first Intramolecular Heck Reaction was discovered by Mori and Ban in 1977. An advantage of intramolecular Heck Reaction is that it can form very hindered carbon-carbon bonds under reasonably mild condition. It has the ability to form quaternary carbon stereocenters with high level of asymmetric induction. The intramolecular Heck Reaction is usually more efficient than intermolecular Heck Reaction due to the elimination of entropic consideration.
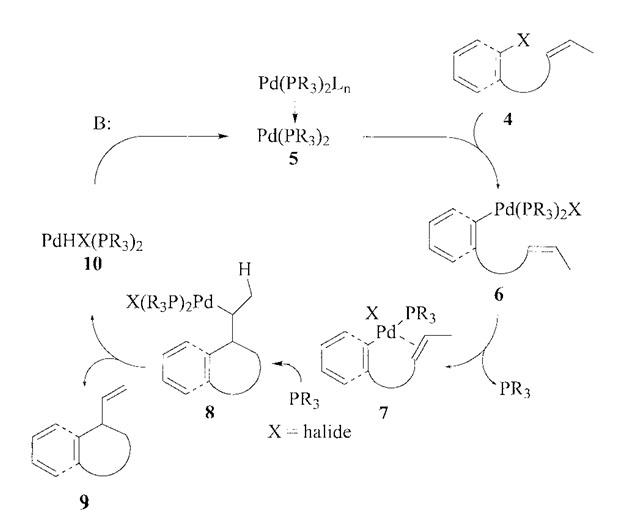
The intermolecular heck reaction uses a palladium-catalyzed coupling of an alkenyl or aryl halide with an alkene to form a substituted alkene. Alkenyl and aryl halides usually react via the neutral pathway, where one of the phosphine ligands leaves the palladium complex during the course of the reaction. The catalytically active palladium generally has two phosphine ligands. The palladium oxidatively attaches to the carbon-halogen bond. The sigma-bonded alkylpalladium intermediate results from the syn insertion of the alkene. The syn β-hydride elimination provides a hydrido palladium complex, and that complex is then converted by a stoichiometric amount of base into the catalytically active palladium complex. In an asymmetric reaction that commonly employs chiral bidentate phosphines, this is commonly used as an explanation for the loss of enantioselectivity, which is often observed in reactions conducted via neutral pathway.
Neutral Pathway